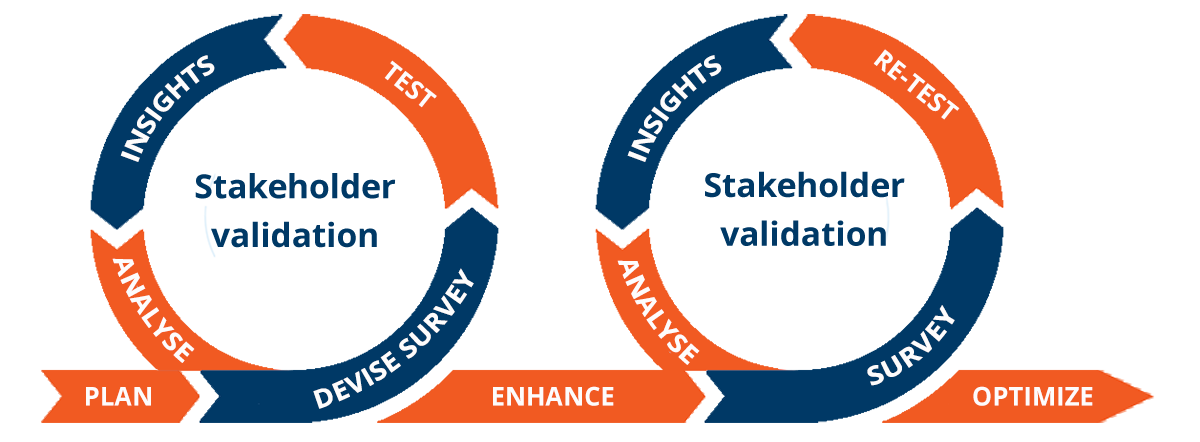
Evidence is a critical part of access decision-making across the lifecycle of a product, with life science companies now needing to take an iterative approach to insight and evidence generation that begins in early development. This increases the efficiency and effectiveness of development and commercialization activities and reduces the time to market.
By combining our HEOR/RWE strategic expertise with RPR, an agile on-demand technology platform that leverages 3000+ global payers, key opinion leaders, patients, and/or regulators, we obtain robust insights from stakeholder and payer engagement faster than ever before and can review, revisit, and adapt evidence strategies at any stage of the process.