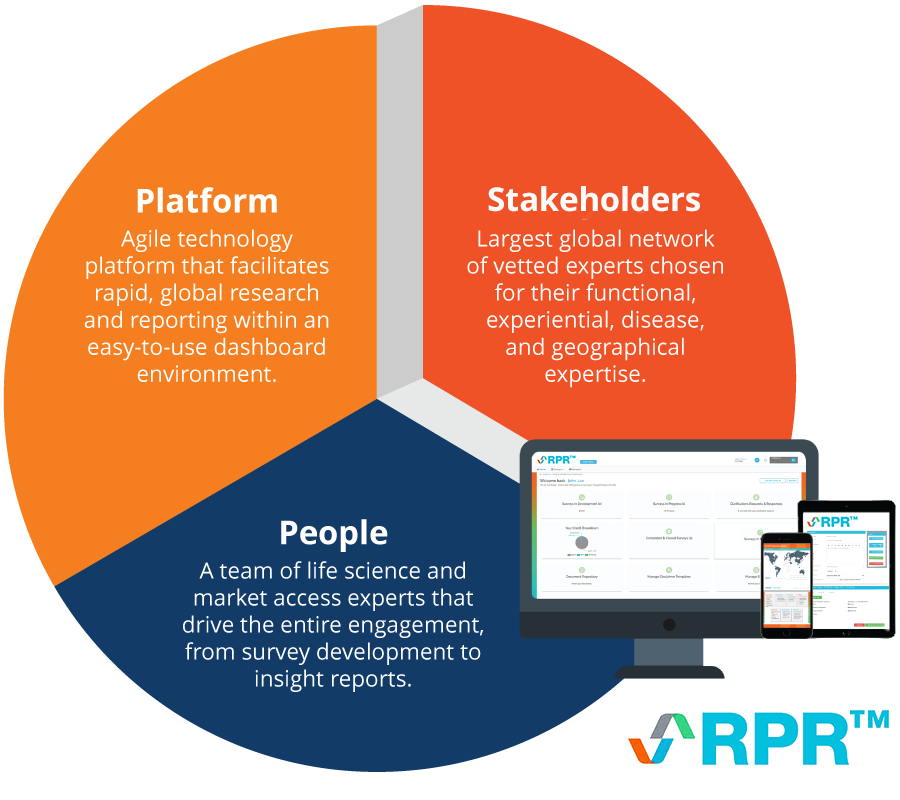
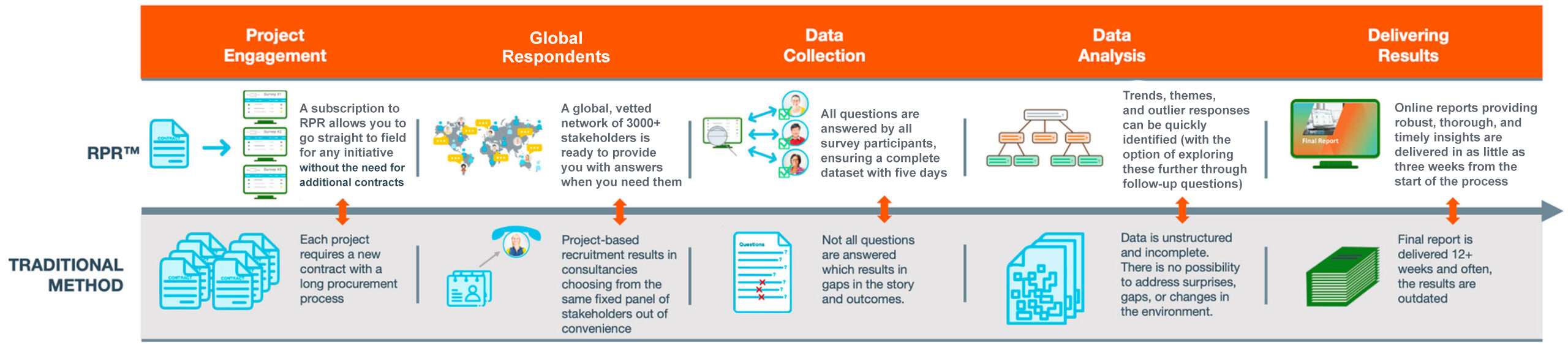
RPR is an agile, on-demand platform that enables faster, higher-quality stakeholder / payer research for market access via direct, automated stakeholder engagement. It offers:
Stakeholder insights
Secure the robust stakeholder insights you need, when you need them
RPR (originally Rapid Payer Response™) is an agile, on-demand technology platform that provides clients with insights via direct stakeholder engagement at a fraction of the time and cost of traditional methods.

RPR - stakeholder insights
3,000
65
3
RPR
Evidence strategy powered by the voice of the stakeholder
- Question development support: A highly qualified team of consultants from the relevant field (market access and pricing, RWE, HEOR, etc.) will develop questions based on your project goals.
- Answers when you need them: Stakeholders submit in-depth answers via an online portal, with responses received in as little as five days.
- Vetted global stakeholder network: Access to a dynamic and growing network of 3000+ stakeholders across 65+ countries, matching specific experts to the geography, objectives, and survey criteria of interest.
- Agile engagement platform: Offers a dynamic ability to engage with stakeholders and conduct both clarificatory and follow-up questions.
- In-depth insights: Specialist teams analyze data collected and provide an in-depth insight report in as little as three weeks, including actionable strategic recommendations.
- Credit-based subscription: Securing credits in advance means projects can be commissioned when in-market expert insight is needed without further time-consuming contracting.
Our global stakeholder network
Over 3000 vetted experts across 65 countries are ready to provide the answers and clarifications you need for stakeholder / payer research via the RPR online platform.
Expert engagement when you need it
We search the globe for the most reputable experts before vetting them for suitability through a rigorous quality assurance and testing procedure. Each expert is tagged and classified according to their area of expertise and location, so that we can match the right experts to the right research objectives for our clients.

Join our stakeholder network
Are you an experienced healthcare decision-maker/stakeholder interested in being part of cutting-edge research that helps patients receive new treatments? If so, consider joining our network and lending your expertise via our online survey platform, only providing input when your schedule allows, and you will be remunerated.
Video
RPR: an introduction
Why RPR?
What are the advantages of an RPR-powered approach to evidence generation, market access, and pricing strategy?
Boosted
relevance
RPR integrates real-world decision-maker insights, enriching research findings and bridging data gaps to align with research goals.
Enhanced
accuracy
RPR not only fills research gaps but also refines insight. For instance, while secondary research might hint at a need for more RWE, RPR pinpoints the methodologies and outcomes acceptable to payers.
Superior
validity
RPR dives deeper than traditional research, sourcing data directly from key audiences, from payers to physicians to patient advocacy.
Strategic
decision-making
Manufacturers can fine-tune their market access strategies with RPR, enabling precise decision-making and clear identification of both risks and opportunities.
A partner on whom you can rely
We have become a trusted market access research partner to top pharma and biotech companies around the world, completing over 700 projects across 45 countries, and securing a 93% renewal rate from our clients.
“RPR... is my go-to resource to gain real-time insights from providers and payers on the value drivers for diagnostics across disparate care areas...”
Head of Global Market Access, GE Healthcare
Looking for a partner to help you engage with in-market experts and enhance stakeholder and payer research for market access?
RPR can also be used to validate HEOR strategy and evidence generation plans: testing and enhancing your approach throughout the lifecycle of a product.


Flexible Integrated Teams (FIT)
Research partnerships reimagined
Our stakeholder engagement solutions can be delivered as individual projects or as part of a FIT engagement, where we partner with you to provide dedicated, cross-functional teams that integrate seamlessly within your own to fill experience gaps or supplement existing resources.
The size and composition of your team can gradually evolve or pivot quickly to meet your changing needs, all without the costly delays involve in adjusting a statement of work.
A trusted extension of your organization, our team becomes your team.
RPR case study
Obtaining insights on a trial design for external control arm in multiple myeloma
A new drug was being investigated in a single arm study to evaluate its efficacy and safety in multiple myeloma participants and there is a need to generate evidence on the standard of care, which could be done by creating an external control arm using real-world data.
RPR was used to obtain key KOL and payer insights on the appropriate study design elements required to create a robust external control arm.
Within three weeks, RPR was able to gather robust insights that allowed clients to identify best and worst practices for designing an ECA and understand that the publication of final results in a peer-reviewed journal was considered the most crucial evidence.
Selected RPR-related publications
Our team members have co-authored over 1000 publications. View a selection of RPR-related publications below or review our other work via our online publications list.
| Title | GR Author(s) | Year | Therapeutic Area | Data Source | Journal/Conference | Full Citation & Links |
|---|---|---|---|---|---|---|
| Assessment of the relevance and credibility of indirect treatment comparisons to help inform health technology assessment and reimbursement decision-making: results of a 5-country payer survey. | Katsoulis I, Graham A | 2023 | General Medicine | RPR Survey | ISPOR Europe | View |
| Paradigm Shift or Incremental Change? Real-World Evidence for Health Technology Assessment in Oncology. | Ignjatovic T | 2023 | Oncology | RPR Survey | ISPOR Europe | View |
Accelerate your insight generation
Our other equally powerful technology platforms play an integral role in our ability to provide life sciences companies with a better way to optimize insights and evidence.

Integrated evidence planning
Tailored precisely to your needs, Integrated Evidence Planning (IEP) platforms provide an intuitive online solution to the complexity of evidence generation planning, management, alignment, and communication across critical stages of the product lifecycle.

AI-powered evidence synthesis
EVID AI is an AI-powered literature review platform that provides the world’s largest, up-to-date database of healthcare literature results and employs machine learning to extract and present the required data within structured evidence tables.

Visualize and communicate RWE
Our interdisciplinary scientific team designs, builds, customizes, and implements fit-for-purpose RWE Dashboards that transform complex data into easily digestible insights that are rigorous, transparent, and impactful.